What is Heat Treatment/Heat Treating?
Heat Treatment and Heat Treating refer to a wide variety of thermal processes applied to a multitude of components that make up almost everything we touch, use, and in many cases consume. In these processes, thermal energy is either added or removed and then returned to ambient /room temperature following a specified time/temperature profile to obtain the desired conditions or properties required for further processing or the final use of the components.
The most common forms of Heat Treating include Hardening and Tempering of Steels, Annealing of metals, Age Hardening of Aluminum Alloys, Curing of Paints, Glues, Varnishes, and Rubber. Petrochemical processes that require heating of reactors and intermediate products along with Precipitation of Chemicals from Oils. But what are these processes and why do we employ them?
Hardening
Hardening means to make a metal more resistant to deformation. Hardness is related to strength, ductility, and wear resistance. During the hardening process, both strength and wear resistance increases, while toughness (the ability to absorb energy and deform plastically before fracture) and ductility (ability to deform plastically without fracture) decrease. Hardening of metals can be accomplished by the following:
- Alloying, also known as the solid solution is usually done at the time that metal is produced. Because alloying commonly increases the cost of a metal, generally manufacturers will select the metal with the lowest alloy content that will meet the needs of the application. Also for many applications, manufactures have found that higher hardness is needed only in select areas where there is high wear or stresses, so processes have been developed for surface alloying of ferrous (meaning the main ingredient is iron) metals. Surface alloying can be accomplished by heating the metal to a suitable elevated temperature and diffusing carbon or nitrogen into the surface, e.g. carburizing, nitriding, and carbonitriding. Surface alloying has the advantage of improving the surface hardness of the metal while maintaining a tough ductile core.
- Cold working (deformation e.g.. rolling, drawing, stamping) – with some metals, such as 3003 aluminum and 304 stainless steel, this is the only way that they can be made harder.
- Precipitation hardening (also called age hardening or particle hardening) is a process where a metal alloy is subjected to a heat-treating process specifically designed for that specific alloy to create particles (precipitates) from a supersaturated (single-phase) solution. Generally, the heat-treating process consists of heating the metal alloy to a temperature that puts all constituents of the alloy into a single-phase solid solution, followed by the metal alloy being quenched to lock all the alloy constituents in a supersaturated solid solution, and finally re-heating the metal alloy back to an elevated temperature for an extended period (hours or days) to allow precipitates to form in the metal alloy, which strengthens the alloy. This process is effective for some non-ferrous alloys (e.g. 2XXX and 7XXX series aluminum alloys, and Inconel, which is a nickel-base alloy) and some grades of stainless steels (e.g. 17-4 PH, 17-7 PH).
- Martensitic transformation (ferrous alloys) – this is the hardening process that people think of when talking about metal hardening, because of its commercial importance. This particular heat-treating process consists of heating the steel/ferrous alloy to above the temperature (Ac3) at which the structure of the steel completely changes from a ferritic structure (body-centered cubic crystal structure) to an austenitic structure (face-centered cubic crystal structure), and then cooling it rapidly so that martensite (body-centered tetragonal crystal structure )forms. The austenite to martensite transformation is diffusionless occurring at approximately the speed of sound. Martensite when polished, etched, and looked at under an optic microscope has an acicular microstructure (needle-like -see Figure 2) that is very hard and brittle, so a martensitic transformation treatment is almost always followed by tempering treatment to reduce the strains in the martensitic structure.
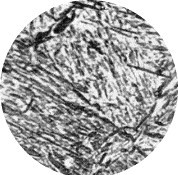
Figure 2: As hardened martensite
The carbon content of commercial steels usually ranges from 0.05% to about 1.0%. Generally, heat treatments to create martensitic transformations are applied to steels having a minimum of 0.3% carbon. Carbon content determines the maximum hardness that can be achieved by martensitic transformation in steels, particularly in plain carbon steels (Fe-C steels). However, as the amount of carbon increases in austenite, the shear strength of austenite goes up and greater undercooling is required to initiate the shear for martensite transformation to occur. In other words, the Ms and Mf temperatures go down, with the Mf temperature dropping below room temperature in alloys containing more than 0.3% carbon. Therefore, significate amounts of untransformed austenite (retained austenite) may be present at room temperature, particularly in high-carbon steels. Above 0.7% carbon, retained austenite has a significant effect on the maximum hardness that can be achieved with plain carbon steel. One way to ensure that these steels achieve their maximum hardness is to subject them to a cryogenic treatment where the steel is quenched in liquid nitrogen (-196⁰C or -320⁰F) or liquid helium (-296⁰C or -450⁰F), to reduce the amount of retained austenite.
Alloying elements also affect the amount of retained austenite that can be present without utilizing a cryogenic treatment.
Some of the ferrous alloys that are commonly hardened by martensitic transformation are carbon steels, alloy steels including Chromoly (AISI 4130) and 52100, cast steel, malleable cast iron, martensitic stainless steels, and tool steels.
Process Details For Martensitic Transformations In Steel
The following are critical temperatures and microstructures that are associated with the steels the metallurgists need to be aware of when hardening steels. Ac1 refers to the temperature at which austenite begins to form in a steel and Ac3 temperature is the temperature at which the steel becomes fully austenitic. These temperatures are determined by the chemistry (carbon content as well as other alloying elements) of the ferrous alloy being heat treated. According to George Krauss in his book, “Steels: Processing, Structure, and Performance” published by ASM International in 2005, Ac3 and Ac1 can be approximated in degrees Celsius by the following formulas: 910
Ac3= 910 – 203√C – 15.2Ni + 44.7Si + 104V + 31.5Mo + 13.1W
Ac1= 723 – 10.7Mn – 16.9Ni + 29.1Si + 16.9Cr + 290As + 6.38W
Note: Typical published Ac1 and Ac3 temperatures are determined using very slow heating and cooling rates. Because transformations that occur at Ac1 and Ac3 temperatures are diffusion-controlled, Ac1 and Ac3 temperatures tend to increase above those associated with equilibrium at rapid heating rates (e.g. induction heating rates) that allow less time for diffusion. Also, at rapid heating rates, steel grain size and structure influence Ac1 and Ac3 temperatures.
Steels that have either a fine pearlite microstructure or a “quenched and tempered” microstructure allow for the most consistent austenitic transformation at rapid heating rates and short heating times, because of the more uniform distribution of the carbon in the microstructure before heating. Steels that have been subjected to a spheroidizing heat treatment before hardening have the poorest response to rapid heating rates.
Also, carbon content and alloying elements determine the critical cooling rate required to get martensitic transformation, instead of pearlite or bainite. Pearlite is a two-phase lamellar (layered) structure consisting of alternating layers of ferrite (solid solution of one or more elements in body-centered cubic iron) and cementite (Fe3C or iron carbide). Bainite is an aggregate of ferrite and cementite that forms at temperatures below where pearlite forms. Upper bainite generally forms at temperatures between 400 – 550⁰C and lower bainite forms at temperatures between 250 – 400⁰C. Upper bainite has a feathery plate-like appearance and lower bainite has an acicular appearance resembling tempered martensite. Martensite transforms in a temperature range Ms (the temperature at the austenite to martensite transformation starts to occur during cooling) to Mf (the temperature at which the austenite to martensite transformation is complete during cooling).
Carburizing
Carburizing is a heat treatment used for adding carbon to the surface of steel parts.
CarboNitriding
Carbonitriding is a heat treatment used for adding both carbon and nitrogen to the surface of steel parts.
Nitriding
Nitriding is a heat treatment used for adding nitrogen to the surface of steel parts.
Flame Hardening
A surface hardening treatment where a torch is used to heat the surface of the steel.
Tempering
Tempering is a thermal process where an as-hardened piece of steel is heated to a temperature below the austenitic transformation temperature to reduce the hardness and improve the toughness of the hardened steel. Generally, a tempering temperature is chosen above the anticipated operating temperature of the component in its final assembly.
Annealing
Annealing is a thermal process that relaxes the material so that it can exist in a more stable condition. It releases strain energy from the material leaving the atomic and crystalline structures at a lower energy state so that they can absorb more energy or work.
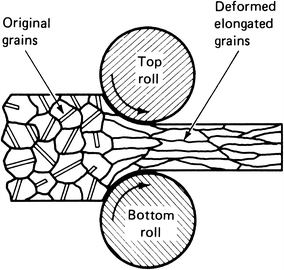
Working a material is exerting force on the material to reshape it in some way, e.g. drawing smaller diameter wire or rod, rolling bar or slab, or other forming processes like forging. Figure 1 illustrates how cold rolling[1] of the metal affects the internal structure of the metal. Original grains represent the fully annealed strain-free internal structure that occurs after an annealing process. The deformed elongated grains represent what happens to the internal structure of a metal during a cold rolling. An illustration of how the grain structure of the metal is affected during drawing of wire or rod looks just like Figure 1 except the rolls would be replaced by a conical draw die and the plane of the illustration would be a cut through the centerline of the die and wire/rod.
Figure 1 – Illustration of how the grain structure of a metal changes during cold-rolling.
Unless specified, annealing refers to full annealing which is where recrystallization occurs and the resulting grains are strain-free and equiaxed (meaning approximately the same dimensions in all directions).
While annealing is used primarily used to soften metallic materials, annealing is also used to produce desired changes in microstructure and properties, such as mechanical and electrical properties, as well as improved dimensional stability.
In general, annealing cycles consist of heating the material system to an elevated temperature, hold for a specified time, and then slowly cooling the material back to room temperature. Ferrous metals are particularly sensitive to cooling rates after annealing because of their ability to experience martensitic transformation if cooled too rapidly. When annealing steel in a furnace, the annealing cycle consists of heating the steel to above the Ac3 temperature, holding at temperature for 1 hour per inch of thickness, followed by slowly cooling the steel in the oven at a controlled rate.
Metals that do not experience phase changes during the annealing process (i.e. C260 brass or 3003 aluminum) can be air-cooled and in some cases force cooled without any detrimental effects.
Examples of Radyne equipment used for annealing are:
- Small Caliber Ammunition Systems
- Large Caliber Ammunition Systems
- Bright Annealing Tube Lines
- Bright Annealing Wire Lines
Normalizing
Normalizing refers to a heat treatment process specific to ferrous metals and is very similar to a steel full annealing cycle. In normalizing, the metal is heated above the Ac3 temperature, held at temperature for some amount of time, then removed from the furnace and cooled in still air.
The only difference between the steel annealing cycle and steel normalizing cycle is that annealing uses a controlling rate performed in a furnace and normalizing is cooled in air.
Spheroidizing
Spheroidizing is an annealing treatment performed on steels with more than 0.8% carbon. The spheroidized annealing treatment consists of heating the steel to a temperature (generally about 1200⁰F (650⁰C)) just below the eutectoid temperature and holding for an extended time to allow the carbon to diffuse and coalesce as spheres of cementite (Fe3C) surrounded by ferrite. Because of the extended time at elevated temperatures, spheroidized annealing is performed in a protective atmosphere (generally endothermic) to prevent oxidation and decarburization.
Spheroidizing is generally done to improve ductility and toughness with reduced strength and hardness, so the steel can be cold worked or machined easier.
Spheroidizing is also known as a subcritical annealing process, meaning the annealing occurs at a temperature below the Ac1 temperature.
Stress Relieving
Stress-relieving is a type of annealing heat treatment; except the peak temperature is relatively low and not high enough for recrystallization to occur. (In the case of steel, stress reliving is generally done below the Ac1 temperature.) As the name implies, this thermal process is designed to reduce the residual stresses in the material. Stress-relieving generally consists of heating to a suitable elevated temperature, holding long enough to relieve residual stresses and then slow cooling back to room temperature to minimize the development of residual stresses. No microstructural phase changes occur during the stress-relieving process.
Stress relieving treatments are commonly used after welding, cold forming processes (i.e. stamping or deep drawing), and machining to eliminate part distortion that can occur during processing.
Fusing
Fusing refers to a thermal process that is used to improve the density and bond of a powdered metal alloy (hardfacing alloy) that has been flame sprayed onto a metal substrate. When fusing, typically the flame sprayed coating is heated to a temperature between the solidus and liquidus temperature of the hardfacing alloy to allow the particles that make up the coating to fuse and eliminate voids in the coating.
Curing
Curing is a thermal process where elevated part temperatures are used to drive off solvents and/or help cross-link polymers in paints, glues, epoxies, varnishes, and other polymeric coatings, e.g. Radyne pipe coating line.